Around 60% of the energy consumption is currently wasted as heat. The use of chemical reactions to store thermal energy is an attractive goal for domestic and industrial applications as heat also comprises around 50% of end energy usage. Utilizing this invisible potential is therefore paramout for the energy sustainable society, crucial for energy transition and is in line with UN sustainable development goals 7 and 11.
Thermochemical Energy Storage (TCES) based on solid-gas chemical reactions is a promising approach to efficiently store heat that may pave the way towards compact and inexpensice devices with high energy storage density (up to 1 GJ/m3) and with absence of thermal losses, thus making possible advanced usage in numerous industrial processes, for seasonal storage, etc. One of the bottlenecks for this technology is lack of appropriate chemical reactions and materials studied for TCES.
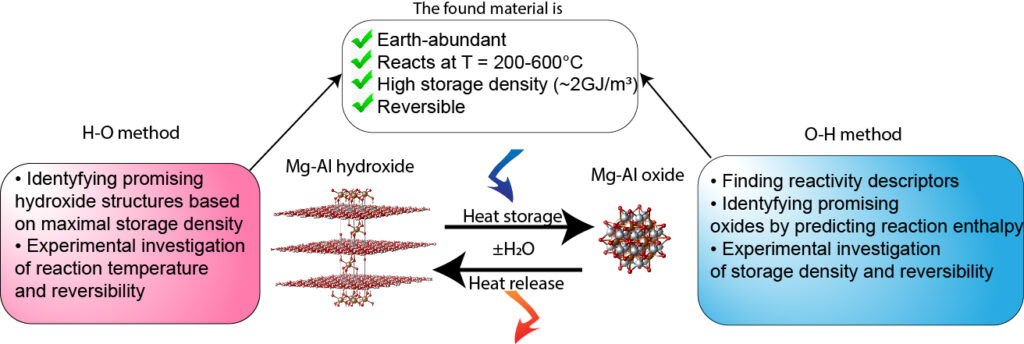
Substantial fraction of the waste heat posesses temperature potential in the medium temperature reason (200-600°C). This industrially relevant temperature interval is very sparsely covered by materials for TCES. Indeed, only decomposition/synthesis Mg(OH)2 (T ~ 350°C), Ca(OH)2 (T ~ 500°C) and MgCO3 (T ~ 400°C) were studied for TCES at medium temperatures. Therefore, the technology lacks flexibility for real use case scenarios.
The project was aimed at extending the material portfolio by sytstematic computationally-aided screening of oxides for TCES. The project contributes to the developing one of the most advanced technologies for thermal energy storage at these temperatures that utilizes the chemical reactions.