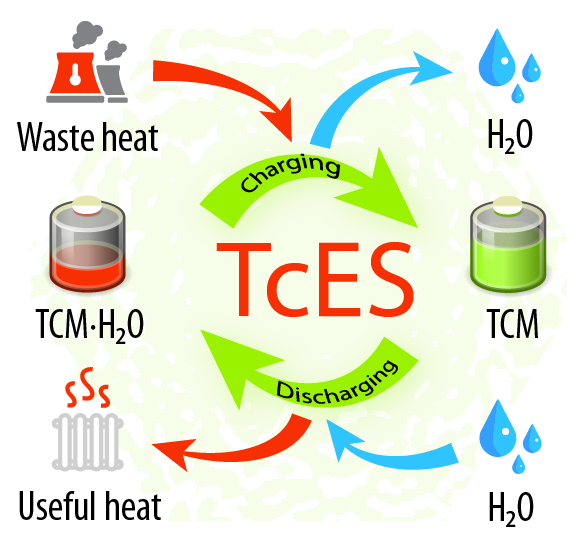
Thermochemical energy storage – that is, storage of thermal energy by means of chemical reactions – is one of the emerging technologies for durable and efficient utilization of heat, providing both high energy storage density and high storage duration. A thermally-driven thermochemical battery utilizes external heat in order to, for instance, endothermically remove water held by a thermochemical material (TCM). As the water is forced to leave a thermochemical material, the waste heat is absorbed thus charging the battery. When the heat is needed, the water vapor is supplied to the charged material in order to initiate the exothermic heat release process.
I seek perfect thermochemical materials which meet criteria of numerous applications – from solar-driven domestic heating to heat storage in nuclear reactors. These days the field is burgeoning with novel materials and approaches for thermochemical energy storage. In the course of my PhD studies I focused my experimental work on elucidating the nature of doping metal oxides with the purpose of controllable reactivity enhancement towards their hydration/dehydration and carbonation/decarbonation. I dedicated my further research to making thermochemical materials (salt hydrates) robust and reliable for domestic applications.